Cucurbiturils
Cucurbiturils are a remarkable class of host compounds often featured by very high binding constants enabling them to form stable inclusion complexes with a myriad of molecules in water. Their shape resembling that of gourds or pumpkins inspired their name given after their discovery in 1981. Today, more and more research groups worldwide work on these fascinating macrocycles. These non-toxic macrocycles are able to encapsulate many drugs thereby improving key properties of medicines like solubility or therapeutic efficacy, but they are also used to capture unpleasant odorant molecules or inversely release fragrances. They are also used to capture gases and to build biological assays, imaging agents, dynamic supramolecular polymers, smart crystals or hybrid materials.
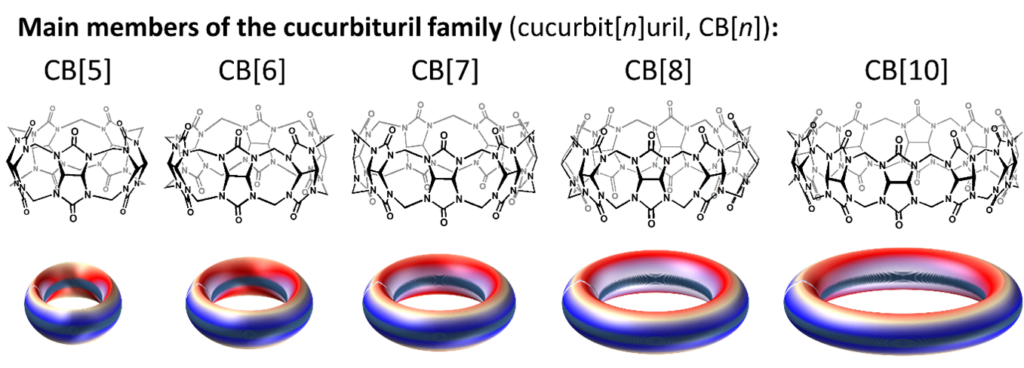
More specifically, here follows our research topics (Anthony Kermagoret, David Bardelang):
1- Artificial molecular switches and machines in water
Since 2015 we have been working on stimuli-reponsive host-guest complexes featuring cucurbiturils. We found that pH-responsive viologens carrying an imidazole function allow for tuning the ring position along a molecular track thanks to pH or silver cations. In parallel, we developed a system enabling guest exchange in the cavity of CB[7] according to a partial energy ratchet mechanism.
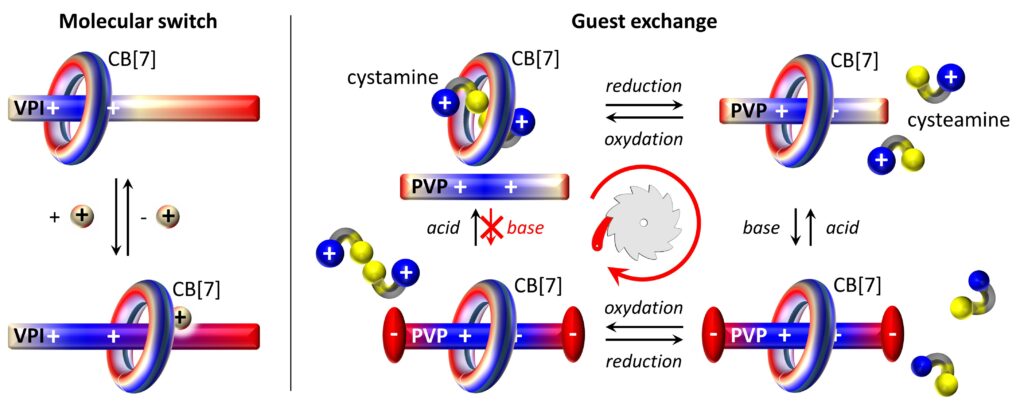
- A pH-driven ring translocation switch against cancer cells, Chem. Commun. 2018, 54, 13825-13828
- Metal actuated ring translocation switches in water, Org. Lett. 2018, 20, 3187-3191
- Guest exchange by a partial energy ratchet in water, Angew. Chem. Int. Ed. 2021, 60, 6617-6623
2- Supramolecular oligomers
The larger cucurbiturils (mainly CB[8] and CB[10]) have opened the way to higher stoichiometry Host-Guest complexes in which the number and orientation of the guest molecules can be modulated enabling to tune a number of structural or physico-chemical properties such as guest pKa, light emission, or conformations. This work is mainly done in collaboratoin with the group of Prof. Ruibing Wang.
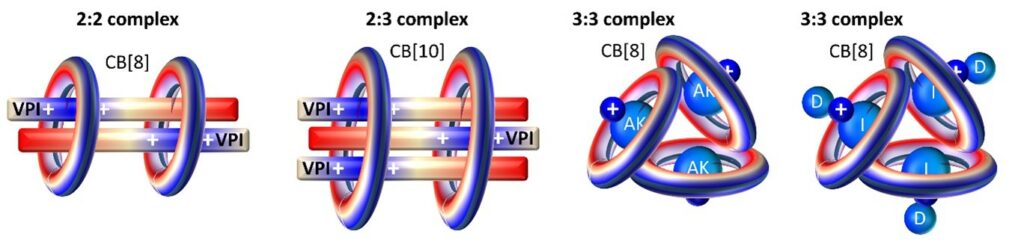
- Triple stack of a viologen derivative in a CB[10] pair, Org. Lett. 2021, 23, 5283-5287
- Triangular regulation of 1:1 cucurbit[8]uril complexes, J. Am. Chem. Soc. 2019, 141, 5897−5907
3- Responsive materials
We are also interested in photochromic materials especially those made from viologens that have the property to become very coloured after light or sun exposure thanks to a photo-induced electron transfer. Single crystals of CB-based MOF were obtained which are photochromic. In parallel, we are working on porous host-guest crystals, a recent example allowing us to capture gaseous iodine with a remarkable selectivity with respect to water without needing energy for material activation.
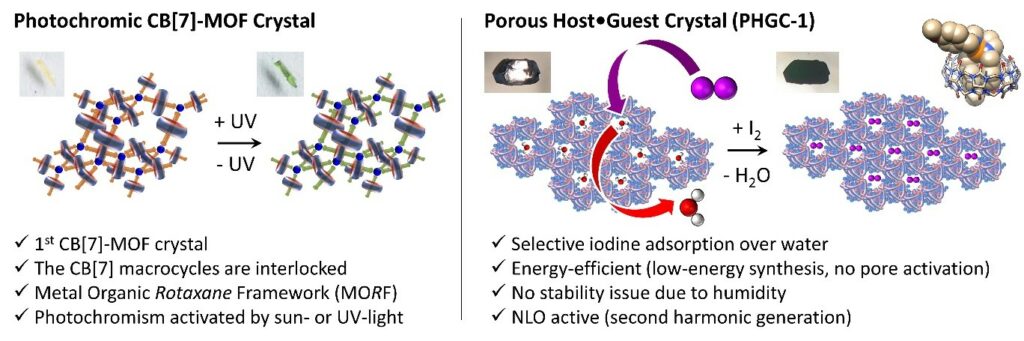
- A single-crystal-to-single-crystal transformation affording photochromic 3D MORF crystals, Chem. Commun. 2019, 55, 13824-13827
- Energy-Efficient Iodine Uptake by a Molecular Host•Guest Crystal, Angew. Chem. Int. Ed. 2022, DOI:10.1002/anie.202214039
4- Toward biological applications
The largest cavity cucurbituril (CB[10]) has enough space to accommodate relatively large molecules. We found, in collaboration with Mike Hannon’s group and Simin Liu’s group that supramolecular (dinuclear triple stranded) helicates can be very effectively included in CB[10]. Using imidazole functionalized helicates, CB[10]-helicate rotaxanes have been prepared thereby affording the 1st switchable supramolecular system in which the binding of a metallodrug (the helicate) toward DNA can be modulated.
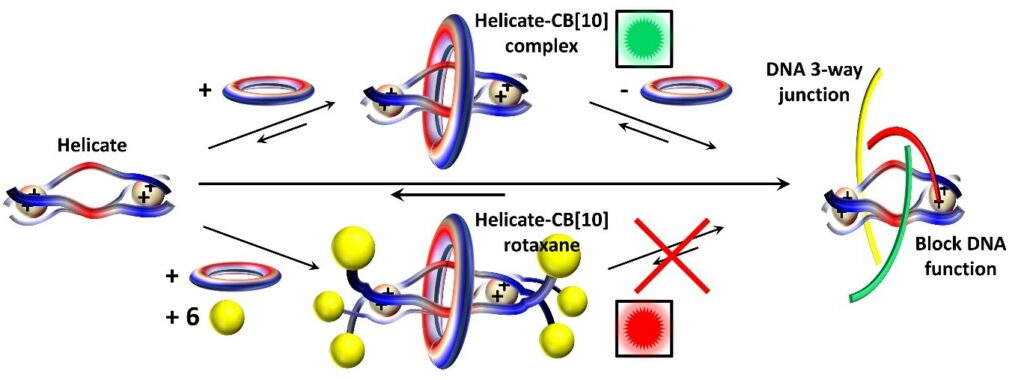
- Rotaxanating metallo-supramolecular nano-cylinder helicates to switch DNA junction binding, J. Am. Chem. Soc. 2020, 142, 20651-20660
